Batteries
A battery is a means of storing electrical energy, usually in a portable form. The
term battery was originally coined by Benjamin Franklin to describe an arrangement
of multiple Leyden jars (a form of capacitor used to store static electricity). Batteries
can be divided into the following categories.
In today’s world all these applications areas are expanding whether it be the demand
for electric cars, or laptops that you can use on a 10 hour transatlantic flight
assuming that the guy in front of you hasn’t put his seat so far back that you can’t
open your notebook…
History of the Battery
It is generally acknowledged that Alessandro Volta at the University of Pavia in
Italy invented the first practical multi-cell battery as a means of generating electricity.
However, ancient devices looking remarkably like batteries have been discovered
near Baghdad. These devices have the characteristics of a battery: two dissimilar
metals separated by an electrolyte. It has been suggested that they might have been
used to electroplate jewelry, but there is no actual evidence. The single-cell battery
was accidentally invented by Luigi Galvani in Bologna in 1786 when he was experimenting
with the nervous systems of frogs (he discovered that touching a nerve with two dissimilar
metals caused the frog’s leg to twitch).
In 1803 Nicholson and Carlisle in England were experimenting with the Volta battery
and discovered electrolysis (the breaking down of molecules by the passage of electricity;
for example, the conversion of water to hydrogen and oxygen). This was the beginning
of electro chemistry that would eventually lead to aluminum smelting, the preparation
of elements like potassium, and electroplating.
In 1803 the German physicist Johann Wilhelm Ritter invented the rechargeable battery.
Sadly, this never caught on at the time because there was no means of recharging
batteries until the generator had been invented.
In 1859 The French inventor Gaston Planté constructed the first practical rechargeable
lead acid battery. This is still used in automobiles today.
In 1866 George Leclanché in France invented the first battery that could be produced
in commercial quantities. The Leclanché cell had a carbon cathode, a zinc anode and
an electrolyte of ammonium chloride solution. The carbon cathode was surrounded by
a depolarizer of manganese dioxide in a porous cylinder. Its output was 1.4 V and
it was widely used during the early days of telegraphy.
In 1881 Carl Gassner invented the zinc-carbon cell. This was the first commercially
successful dry cell battery. Up to this point all batteries involved liquids.
In 1900 Thomas Edison in the USA invented the nickel-cadmium rechargeable battery.
In 1949 the Canadian Lew Urry invented the alkaline battery, although its later versions
did not go into large scale production until 1968. This is the type of battery widely
used today to power small portable devices (when rechargeable batteries are not used).
In 1979 lithium-ion technology was perfected by John B. Goodenough. This is the basis
of batteries in today’s laptops, cell phones, tablets, GPS, and digital cameras.
In 2013 the entire fleet of new Boeing 787 Dreamliners was grounded because of a
fire in a lithium cell. Modern battery technology has been so successful that it
has been incorporated in new aircraft to reduce weight. A series of incidents involving
lithium-ion batteries (one incident was a fire) led to the grounding of an entire
fleet. This demonstrated that battery technology still has some way to go.
How Batteries Work
A cell is a device that generates electricity by chemical means. If we stack cells
together in series (daisy-chaining them together with positive terminal to negative
terminal), we get a battery, although in common usage the term battery is used to
describe devices with just a single cell. In general, the potential difference across
the terminals of a cell is about 1.2 to 3.6 V and larger voltage are created by stacking
cells in series; for example, a 9 V battery uses six 1.5 V carbon-zinc cells in series.
A cell consists of three components: a cathode which is the positive pole or terminal
of the cell, an anode which is the negative pole of the cell, and an electrolyte
which fills the space between anode and cathode and which conducts ions between the
anode and cathode. An ion is an electrically charged atom.
A chemical reaction takes place at the anode which causes electrons to flow out of
the anode into the external circuit and then back into the cathode. The battery is,
therefore, an electron pump. Positive ions move through the electrolyte from the
anode to the cathode. At the cathode the negative electrons flowing in from the external
circuit meet the positive ions flowing through the electrolyte where they neutralize
each other.
The difference between batteries lies entirely in the form of the anode, cathode
and electrolyte. Consider the following examples.
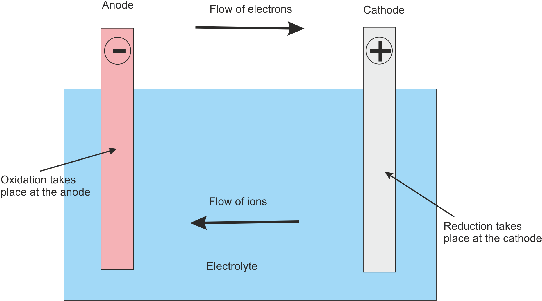
The following diagram illustrates the operation of a cell. An oxidation reaction
takes place at the anode and the released electrons travel via the external circuit
to the cathode where a corresponding reduction reaction takes place.
Battery Characteristics
The specification of the ideal battery is simple: Its size and weight should be zero.
You don’t really want to lug that laptop around, do you? Its cost should be zero.
It should have an infinite capacity. Who wants to be in the Gobi desert when their
MP3 player stops working? It should recharge instantaneously. It should also be
able to deliver large power surges. A few milliamps will run your digital camera,
but you need rather more current to start your Mercedes on a cold morning. Its output
voltage should be constant and not decay throughout its life. It should have a low
self-discharge current; that is, if you leave it lying around for a hundred years
or so, it should still be as good as the day you bought it. Finally, it should be
reliable and show no inclination to burst into flame. In practice, we don’t usually
get what we want.
Consider the lead-acid cell. It’s really quite horrid. It uses liquid acid in an
unsealed container and it gives of hydrogen gas. It is very heavy and the energy
stored per unit weight is one of the lowest of all batteries. However, it is very
cheap and can supply a the massive current (e.g., 450 A) needed to supply the starter
motor in an automobile.
Most batteries used to power laptops and similar devices use lithium ion technology.
The energy density of a battery is measured in watt hours per kilogram (Wh/Kg). A
battery’s energy density is largely determined by the material of its cathode; a
lithium nickel cobalt cathode has an energy density of 240 Wh/kg which makes lithium
ion technology the first choice for designers.
History of Electronics
Semiconductors
Electronic Circuits
